Search Results: Tonya McKay

Mineral oil has been found in cosmetic and personal care products for 100 years or more, because it is an excellent lubricant that is lightweight and non-greasy. However, in recent years it has really fallen out of favor with consumers for a variety of reasons, among them being concerns about safety. It may also have been a victim of negative marketing simply because it is unpretentious and cheap, which is not necessarily advantageous to companies spending time and money developing new materials for hair care. Let’s take a closer look at mineral oil and see what it is, where it comes from, and what it does for hair, so we can make informed decisions without the influence of marketing agendas.
What is Mineral Oil?
Mineral oil is a mixture of simple hydrocarbon molecules of varying molecular weight derived from the petroleum cracking process. It is a cheap byproduct found to be easily purified and useful in a variety of applications for which a lubricant is needed. Mineral oil is a mixture of medium-to-long chain alkanes (15 – 40 carbons”> with the general formula of CnH2n+2. There are no other elements present in mineral oil. The molecular structure of these materials is very uncomplicated, extremely stable and nonreactive.
Petroleum Cracking
Crude petroleum is a huge organic soup, containing many different carbon-based molecules of varying molecular weights. The petroleum cracking process uses thermal and other catalytic means to break these molecules down into lighter, smaller molecules such as octanes, which are highly desirable as fuel because they are easily combustible and fairly efficient. Other components, such as larger hydrocarbon molecules that comprise mineral oil, paraffin wax, and petroleum jelly, are byproducts of this process and are separated out via distillation. More dangerous byproducts of the petroleum cracking process, such as benzene, are easily separated out due to the large differences in molecular weight.
Safety
As a byproduct of a necessary process for energy applications, mineral oil is an extremely cheap additive which of course gives it favor with formulators striving to increase profit margins on their products. It is not a toxic material and is considered to be an extremely safe and effective skin care and hair care ingredient. It has really gotten a bad rap on the internet and in certain marketing campaigns, but the empirical evidence and data simply do not support this.
Cosmetics grade mineral oil undergoes a rigorous purification process prior to being sold as a raw material and contains no residual dangerous components of the petroleum cracking process. The molecules of which it is composed are inert, non-toxic, and nonreactive, which makes them safe for use in external applications. (There are some risks associated with internal consumption of mineral oil, because it can block nutrient absorption.”>
Mineral Oil for Hair
Mineral oil is a good lubricant, and thus performs well as a detangler. It deposits onto the surface of hair strands and forms films that are sufficiently thick to mask irregularities in the cuticle structure, which gives it fantastic smoothing and emollient properties. It significantly decreases wet combing forces and can help prevent breakage. The film formed by mineral oil on hair is occlusive, meaning it prevents the passage of water through it in either direction. Thus, it acts as a protective barrier that aids in moisture retention by preventing the diffusion of water from the interior of the hair out into the environment in dry conditions, and it also helps to minimize frizz by preventing penetration of moisture into the hair in humid conditions. It has also been found to minimize damage to hair caused by chemical relaxers, so it is often included in those products.
Mineral oil is effective at enhancing curl formation and curl clumping as well. It is able to do this because of the films it forms on the surface of hair fibers. The films exert capillary forces between adjacent hair strands, which causes them to be attracted to and stick to one another. This phenomenon is known as capillary adhesion. The capillary adhesion for mineral oil remains fairly constant over time, so if you can manage to not handle your hair very much, it can help with curl retention throughout the day.
Mineral oil is completely hydrophobic (water-hating”>, and for that reason needs to be washed with a surfactant-containing shampoo. It is easily removed though, and does not require harsh products. Something that contains mild surfactants such as sodium cocoyl isethionate or cocamidopropyl betaine should be completely fine. Another thing to be aware of is that dirt is often attracted to these simple organic oils, and so second or third day hair may begin to look a bit unkempt, depending upon your environment.
Conclusion
It is my opinion that mineral oil is not one of those materials that must be avoided at all costs. It provides some pretty decent benefits, and I would not personally discard a potential product simply because it was on the ingredients list. It looks as if it is especially useful if you live in very dry or very damp climates or if you use chemical relaxers of any sort. It certainly may not deliver the same level of performance as some vegetable oils or even some of the synthetic polymer emollients, but it certainly can be adequate.
References:
J Cosmet Sci. 2007 Mar-Apr;58(2″>:135-45. Effect of oil films on moisture vapor absorption on human hair. Keis K, Huemmer CL, Kamath YK.TRI/Princeton, Box 625, Princeton, NJ 08542, USA.
J Cosmet Sci. 2003 Mar-Apr;54(2″>:175-92.Effect of mineral oil, sunflower oil, and coconut oil on prevention of hair damage. Rele AS, Mohile RB.Research and Development Department, Nature Care Division, Marico

PQ-68
Some of you may know that my main passion in the world of chemistry and materials is for polymers. I frequently peruse the literature looking for interesting new polymers or old ones being used in new applications, especially in the field of hair and skin care. Recently, I was doing some research on a few newer cationic polymers (of the INCI : polyquaternium-xx family”>, when I ran into one that I found fascinating, especially for curly-haired consumers. Its industry trade name is Luviquat Supreme, and it is manufactured and distributed by BASF Corporation. The INCI designation for this polymer is Polyquaternium-68 (PQ-68″>.
The polymer nerd in me was intrigued by the complex structure of this molecule (see below”>. It is a synthetic quaternized copolymer comprised of 4 different monomers (vinylpyrrolidone (VP”>, methacrylamide (MAM”>, vinylimidazole (VI”>, and quaternized vinylimidazole (QVI”>”>, with an average molecular weight of around 300,000 grams/mole. It has positive charges along the polymer backbone, which give it the ability to spread easily onto the surface of hair and form a film that adheres to it. Its physical structure is such that it is a very stiff molecule. When polyquaternium-68 is applied to hair in a styling product, the resultant encapsulating film smooths the cuticle, providing mild conditioning properties, and also imparts body and structure (style”> to the hair.
The inflexible molecular structure of Luviquat Supreme gives the polymer a very high degree of stiffness (measured on films and reported as Young’s modulus”>. This property allows it to impart very strong hold to hair. The inherent rigidity of PQ-68 means that significantly less polymer is required in a product to get the same amount of hold as other common styling polymers such as PQ-4, PQ-11, which makes using the specialty polymer more affordable. Formulators can also tweak the amount of hold their product provides, simply by adjusting the quantity of polymer in the formulation or by adding small molecule ingredients used to soften the films (called plasticizers”>.
Luviquat Supreme is particularly advantageous when used in styling products designed for curly hair. Its physical properties make it excellent at curl formation as well as curl retention. Due to its chemical structure, it provides nearly 100% hold and curl retention in extremely high humidity, and far outstrips the performance of other traditionally used styling polymers (tested at 90% RH, compared to PQ-4, PQ-11, PQ-55, and PVA/VP”>. It also does not get that sticky, tacky feeling that many other polymers get in humid conditions. An additional benefit to curlies is that, despite its outstanding moisture resistance in humid weather, PQ-68 is completely soluble in water. So it is extremely friendly to low-shampoo and no-shampoo routines!
A Synergistic Combination
When a film formed by a styling polymer is too stiff, it can feel unpleasant and yield a sensation of “crunchiness” which some consumers dislike. Also, a very stiff film is typically brittle and can break into tiny pieces, resulting in an annoying flaking problem common to many gels and mousses. In an effort to remedy this drawback, small molecules such as propylene glycol and panthenol are often added to styling products. Their presence in the composition acts to soften the film formed by the polymer. However, in formulation, there is often a give and take when combining ingredients, and the addition of an ingredient to achieve a desirable property often results in a new undesirable property or loss of performance. It is a tricky business.
Well-aware of this formulating conundrum, researchers at BASF were pleasantly surprised by properties obtained when they combined PQ-68 with panthenol. Not only did the panthenol soften the film and add shine to the hair, but it also enhanced some physical properties while preserving many of the original desirable properties. When they performed tensile testing, they found that the flat film stiffness was decreased as expected, but that the elasticity of the film was exponentially increased. Similar testing was performed on treated swatches of hair, and the data showed that the hair strand stiffness was not significantly affected, but that curl retention was enhanced. Remarkably, the presence of panthenol in the films did not deteriorate the excellent humidity resistance of the product.
What this means is that this PQ-68 + panthenol combination allows for exceptional curl formation, curl hold, and curl retention. When a curl is deformed by touching it, by wind forces, or by wearing a hat or scarf, a brittle film might break and the style will be lost. However, a highly elastic film will bounce back when the force is removed. This is exactly what researchers found to happen with this ingredient combination. The same experiment performed with other polyquaternium materials plus panthenol did not produce the same results, so there is something unique going on between these two molecules.
The Polyquaternium-68/ Panthenol combination is found in the following products:
- Fekkai, “MORE” All-day density styling whip, formulation appears fairly CG friendly
- Rene Furterer Volumea Volumizing Mousse, formulation may be potentially drying due to higher quantities of alcohol
- Zero Frizz Curl Rescue Perfecting Spray
- David Babaii for WildAid, Amplifying Whipped Mousse—formula contains many natural oils in addition to the “magic combo” and looks very promising
- Nivea, Flexible Curls Styling Mousse
Clearly, this ingredient combination seems to work well in mousses and styling foams. There are several sample formulations provided at the raw material manufacturer’s site (BASF”> for making them. However, I would like to see the polymer used in a gel product, as that is a styling product that seems to be preferred by many of us with curly hair . I was wondering if that was possible due to potential gel clarity or water solubility issues, and then I found this gem: Hair styling composition, USPTO Patent Application 20090226390
In this 2009 patent application, the inventors make a number of claims for incorporation of PQ-68 and PQ-4 into a clear, sprayable styling gel. The descriptions states that an aqueous-based, alcohol-free, formulation containing a mixture of polyquaternium-68 and polyquaternium-4 formed a sprayable gel with a high degree of clarity. This gel was found to spread most easily on damp or wet hair, which facilitated adhesion between hairs. The composition provided firm, yet flexible hold, that was long-lasting and resistant to humidity. It sounds perfect! I have yet to locate a commercially-available gel product with this composition, but I expect (hope”> we will be seeing it soon (especially once this patent application is approved”>.
I think that the polymer research team at BASF has really hit upon an unexpected winner in polyquaternium-68 (Luviquat Supreme”>, especially for those of us with curly hair. I hope to see this ingredient, along with panthenol, included in more curly hair styling products as hair care formulators start to experiment with it.

Ximenia Americana
I just love the current trend of blending exotic botanical oils with synthetic ingredients to create truly modern, innovative products. In many cases, these types of formulations achieve improved performance over both “all-natural” and “all-synthetic” types of products. Another benefit of incorporating these botanical oils into products is that they are often obtained via fair trade practices and provide an important economic boost to the local economies from which they are harvested and processed. Solvent-free processes are also the preferred method for extracting these oils, which makes them an environmentally friendly choice. Two of the more unusual exotic oils that are being incorporated into products now are ximenia oil and argan oil.

Ximenia fruit
Ximenia Oil
Ximenia oil is obtained from the seed kernel of the Ximenia Americana, a small tree or shrub found in the woodlands and savannahs of Southern Africa ranging from Namibia to Zimbabwe. The trees bear fruit known as monkey plums, sour plums, or wild plums. Extraction of the oil can be accomplished using various techniques, but commercially available ximenia oil is usually processed using eco-friendly, solvent- and GMO-free methods.
The fatty acid composition of ximenia oil is dominated by monounsaturated fatty acids, with the major component being oleic acid. The remainder of the fatty acids are longer chain monounsaturated fatty acids and a small percentage of medium chain saturated fatty acids. This molecular composition of ximenia oil makes it a very attractive ingredient for use in products such as skin moisturizers, hair conditioning products, and hair styling agents.

oleic acid
Oleic acid, which comprises approximately 50% of the oil, is a shorter chain fatty acid that can penetrate into the cuticle of hair strands, as can stearic and palmitic acid. The long chain monounsaturated fatty acid molecules remain on the surface of hair, and make hair soft, shiny, and easier to comb. As they are organic fatty acids, they can attract dirt to the hair and so should be removed more often than some of the newer synthetic polymers, such as silicones and fluoropolymers, which repel dirt. The good news is that although these fatty acids are not water soluble, they are sufficiently small molecules that conditioner washing or washing with mild shampoos should be effective in removing them from the hair.
Argan oil
Argan oil is obtained from the fruit of the ancient Argania Spinosa, a tree indigenous to the arid climate of southwestern Morocco. The tree, which dates back to the Tertiary period (about 1.5 million years ago”>, is an invaluable component of the local ecosystem, as it helps prevent erosion due to wind and rain, and protects the area from the ever-encroaching Sahara desert. Argan oil has been harvested and used in the region for thousands of years for both cosmetic and culinary purposes. Sadly, in recent years there has been a dramatic decline in the Argan tree population due to both the chopping down of trees for firewood and over-grazing of goat herds.

Argania Spinosa
Fortunately, the formation of cooperatives by Berber women has resulted in an international endeavor to protect and save this very important natural resource. The cooperatives are aimed at educating the local populace as to the value and import of the Argan tree as well as making a concerted effort to commercialize of Argan oil. The oil is extracted using organically-certified eco-friendly techniques and is done using a sustainable business model. The monies obtained by the cooperative go back into the community to provide education for tribal women and children, to contribute toward reforestation, and to benefit the rural Moroccan socioeconomic structure as a whole.
Argan oil is a unique composition of fatty acids, proteins, polyphenolics (antioxidants”>, tocopherol (Vitamin E”>, phytosterols, and a rare component – squalene. This mixture of active substances gives argan oil many beneficial properties for both skin and hair. The fatty acid content of Argan oil is primarily (~80%”> a mixture of oleic acid (monounsaturated fatty acid”> and linoleic acid (polyunsaturated fatty acid”>, with two saturated fatty acids making up the other 20%. when Argan oil is incorporated into a hair conditioner or styling product, the monounsaturated fatty acid component (oleic”> and the medium chain saturated fatty acids are able to penetrate the cuticle, which helps increase the suppleness and elasticity of the hair. The polyunsaturated fatty acid remains on the surface of the hair, where it is able to provide emollience and softness. A current product manufacturer has shared with NaturallyCurly.com that they are combining Argan oil with silicone in their products, and that the silicone acts as a seal on the exterior of the hair to keep the Argan oil inside the hair, where it can provide longer-lasting benefits. This is a fascinating application of mixing modern ingredients with ancient ones in order to achieve a superior product.
What should we expect from these oils?
Both ximenia and argan oil have very similar fatty acid profiles to mango butter, avocado oil, and olive oil, all of which are valued for their ability to soften dry tresses. For this reason, if you have experienced success with those oils on your hair or skin, you may expect to see similar results from ximenia and argan oil. However, as we discussed in a previous article (Butters and Oils“>, if your hair is extremely porous, you may experience unpleasant effects from products containing these oils, as your hair may absorb too much of them. So if you enjoy these oils or products that contain them, just try using a little less quantity if you encounter problems such as frizz or unpleasant texture to your hair. If either of these work well for you and you can make your own products or find a ready-crafted product that you enjoy, you can not only enjoy your silky hair, but you can also feel satisfied that you are contributing to the local environment, economy, and social structure of several indigenous tribes in Africa.
Table: Comparison of Fatty acid composition of Ximenia and Argan Oils to other common botanical oils
| Vegetable Butter or Oil | Fatty Acids (total may be <100%"> | ||||
|---|---|---|---|---|---|
| Ximenia Oil | Oleic acid
Monounsaturated 45-55% |
Octacosenoic, Tetracosenoic acid
Monounsaturated long chain 8-12% each |
Hexacosenoic acid
Monounsaturated Long chain 6-8% |
Cerotic, Lignoceric, Linolenic,Linoleic, Erucic Acids
Monounsaturated fatty acids Long chain 1-3% each |
Stearic acid, Palmitic acid
saturated 1-3% each |
| Argan Oil | Oleic acid
Monounsaturated 43% |
Linoleic acid
Polyunsaturated 37% |
Palmitic acid
Saturated 12% |
Stearic acid
Saturated 6% |
|
| Mango Butter | Oleic acid
Monounsaturated 40-50% |
Linoleic acid
Polyunsaturated 5-8% |
Palmitic acid
Saturated 5-8% |
Stearic acid
Saturated 5-8% |
Arachidic
saturated 1-4% |
| Shea Butter | Stearic acid
Saturated 85-90% |
Oleic acid
Monounsaturated 5-10% |
Palmitic acid
Saturated minor component |
Linoliec acid
Polyunaturated long chain minor component |
Arachidic
saturated long chain minor component |
| Avocado Oil | Oleic acid
Monounsaturated 55-75% |
Palmitic acid
Saturated 9-20% |
Linoleic acid
Polyunsaturated long chain 10-15% |
Palmitoleic acid
Monounsaturated 2-10% |
Stearic acid
saturated long chain 0.1-2% |
| Coconut Oil | Lauric acid
Saturated shorter chain 45% |
Myristic acid
saturated 17% |
Palmitic acid
Saturated 8% |
Caprylic acid
saturated 8% |
Linoleic acid & oleic acid
unsaturated 5-10% total |
| Olive Oil | Oleic Acid
Monounsaturated 55-85% |
Linoleic acid
Polyunsaturated 9% |
Linoleic acid
Polyunsaturated 2% |
||
| Jojoba Oil | Eicosenoic acid
Monounsaturated 69% |
Erucic acid
Monounsaturated 16% |
Oleic acid
Monounsaturated 10% |
||
| Almond Oil | Oleic Acid
Monounsaturated 65% |
Linoleic acid
Polyunsaturated 25% |
Palmitic acid
Saturated 6-7% |
Stearic acid
Saturated 1-2% |

Natural, organic, healthy, naturally-derived, plant-based, renewable, green, pure: Close your eyes and throw a dart inside a beauty supply store and you will be hard-pressed to not hit a target with at least one of these buzz words on its label. The current marketing trend is so prevalent that it is a rare personal care product indeed that doesn’t make some sort of claim to be healthier, more natural, better for you or the environment. We are inundated with a plethora of information via all forms of media regarding the way ingredients in products may affect our health and well-being.
All of this hype can lead to people feeling very uneasy and even outright fearful, and product development and sales people are more than happy to capitalize on those concerns. The result can be high-quality new products becoming available to the consumer, but it can also be labels loaded with misleading information, false claims, and even real duds in terms of performance. The buyer must be savvy. In a series of articles, I will be addressing a few topics related to this green marketing campaign and do some educating and myth-busting . This month I will discuss soaps in shampoos and skin cleansers.
Shampoos and skin cleansers are a huge sector of the personal care market. In recent years, there has been a real (perhaps not entirely undeserved”> backlash against products that contain sodium lauryl sulfate and other similar synthetic surfactants, so there has been a huge research and development push in the industry to provide more mild and “natural” products. Those of us with delicate, curly hair are always on the prowl for products that will cleanse well, but leave our locks healthy and silky. So in many ways this is a fantastic trend for us. A wider variety of products are now available that contain milder surfactants than the sulfates and that also contain plant oils and emollients that help restore moisture to the hair.
However, there is one ingredient that is a great example of how natural and old-fashioned is not always an improvement. I am referring to soap-based shampoo bars and shampoos that contain ingredients called “plant oil soaps” or “organic saponified plant oil.” In past articles, we discussed the chemical nature of soap molecules, soap bars, and how they work and the potential problems they present for curly hair (Shampoo and soap bars, Porosity and Curly Hair”>. Please have a look at those if you would like a more in-depth discussion about soaps.
To briefly review, soap molecules are surfactants made from reacting natural fats (triglycerides from animal or plant sources”> with a very strong base (usually sodium hydroxide (lye”> or potassium hydroxide (potash”>”> to form ionized, alkaline fatty acids. This reaction process is called saponification, and while it has been used by humans for many centuries to make soaps, it really isn’t any more natural than many other forms of synthesis that take place in a laboratory. Saponification is a rather environmentally friendly process, though, so soaps can be set apart from the detergent crowd for that reason.
Soap molecules are anionic surfactants (just like sodium lauryl sulfate, but with a different head group”>, materials that have both hydrophilic (water-loving”> and hydrophobic (water-hating”> moieties, and as such are reasonably effective at removing grease and oil from hair, skin, and clothing fibers. However, they come with a set of complications that do not occur with most other synthetic surfactants. A brief summary of the drawbacks of these soap molecules is that they have a much higher pH than is ideal for hair, and permanent damage to the cuticle and lipid layer of hair strands can occur when these products are used. They also react with minerals found in hard water to form an unpleasant film of soap scum and mineral scale on the hair, which can lead to a rough texture, tangling, breakage, and a general dry sensation. Hard water also significant affects the cleansing efficacy of soaps. For these reasons and more, synthetic surfactants are used in commercial products more often than soaps.
How and Why are Soaps in my shampoo or facial cleanser?
Typically we think of soaps as being solids, but it is possible to dissolve solid soap into an aqueous solution to form a liquid shampoo or skin cleanser formulation. Another way to do it would be to take the neat soap solution that is the product of the saponification reaction and add excess water and other ingredients directly to that mixture. The primary difference between liquid and solid soap is that liquid soap is made via reaction with potassium hydroxide rather than with sodium hydroxide. The reason for this is due to the larger atomic size of potassium which enables the molecules to remain further away from one another in solution, thus preventing flocculation and precipitation. A pure liquid soap is clear, but most products have additives such as fragrance, viscosity modifiers, pearlizers, emollients, emulsifiers, and preservatives.
The ingredients list of a shampoo or skin cleanser will indicate the presence of soap molecules using terms such as “olive oil soap”, “coconut oil soap”, “corn oil soap”, “soap of jojoba oil”, or “organic saponified avocado oil”. These are not specifically approved INCI terms for these ingredients, but they do make it pretty easy to spot them in a product. Liquid cleansers and shampoos that contain soap molecules will have most of the same drawbacks of a soap bar, but may be more gentle simply due to being less concentrated.
Take-home message?
Soaps are so appealing on a natural and health-conscious level, as they are made with many fewer chemicals using natural plant oils in a very environmentally friendly process. Unfortunately, they do have many drawbacks in terms of performance, and there are other naturally-derived cleansers that are less harsh for curly hair. However, if the soap is listed lower down the ingredients list (meaning it truly is a minor component of the product”>, the pH of the product may be lower (more acidic”> and thus the product may not be less harsh than one with a high concentration of soap. If the rest of the product looks appealing to you, it might be worth trying it to see how it works for you. I always feel the best data is obtained by the end user when they experiment with a product on their own hair, so don’t be afraid to try something new if it looks like the soap is not the major component.

There has been a lot of buzz in the beauty trenches the past year or so about the new product line released by a newcomer to the personal care product industry, Living Proof. The academic pedigrees, business experience, and sheer intellectual prowess of the founding minds and in-house research team of this start-up is nothing short of dizzying, especially when one considers the size of the company. The board is composed of a brilliant team of scientists from MIT, investors and idea generators from Polaris Venture Partners, and two extremely talented and experienced stylists.
Living Proof co-founder Jon Flint (partner in Polaris Venture Partners”> has a passion for investing in and developing new business ventures in all areas of technology and consumer products. He is always on the lookout for new ideas. Conversations held with prominent stylists Ward Stegerhoek and Mitch DeRosa about the current state of haircare and skincare products led to the inception of the concept of Living Proof. Their vision was to assemble a multidisciplinary team of scientists from the medical, chemical, and biotechnical fields to work in concert with top stylists to develop truly innovative products for skin and hair.
None of the scientists who were part of the original team had any experience in the personal care product development industry, but were all extremely prestigious in their own areas of expertise. Although this is stated nowhere overtly in the Living Proof literature, I believe this was purposeful, as their goal was to approach product development from a foundational, molecular level with a specific goal, and to do so without any of the baggage that might be associated with a common formulator mindset of, “but this is how you must design a hair conditioner, because this is how everyone designs a conditioner.”
Breakthrough
The goal of the research and development team was to develop a truly novel product that would work to help control the universal problem of frizzy hair, especially in humid conditions. In order to have smooth locks, it is essential to have a hair product that will seal the cuticle of the hair shaft in order to prevent water from passing into and out of the hair. The product must be able to accomplish this task without weighing the hair down or making it greasy. (For more detailed information on hair, humidity, and frizz, review these relevant articles. Humidity, Humectants, and the Hair, Dew Point, Porosity“>
People have relied upon oils and serums throughout history to tame their tresses, but have had to deal with greasy, unpleasant feeling hair that may have even still been frizzy. Many of these products were made of plant oils, animal fats, mineral oil, or fatty alcohols. Silicone oils were a revolutionary discovery in the latter half of the twentieth century, and became the go-to ingredient for anti-frizz serums and products. However, even these space-age polymers were not without drawbacks. Many consumers found themselves dealing with hair that became increasingly more unruly, lank, greasy, and even dry. This is especially the case for curly haired consumers, whose delicate locks seem to be particularly susceptible to the limitations of silicone polymers.
The scientists at Living Proof reasoned that one of the limitations of silicone polymers in hair care applications is their relatively large molecular size. These large molecules sit on the surface of the hair, can attract dirt and particles, and weigh the hair down. For this reason, it is recommended that silicone serums and products containing silicones be used in relatively small amounts. However, this becomes a drawback itself, as there ends up being incomplete coating of the hair by the silicone, and thus some hair strands are left vulnerable to the problems of ruffled cuticle, frizz, and tangling caused by exposure to humidity.
It became clear that in order to be truly on the cutting edge, it would be necessary to develop an entirely new kind of polymer that could conquer the problem of frizz by coating the entire surface of all of the hair, but that would not create its own set of problems. After a year of intensive laboratory and literature research, the Living Proof scientists discovered the polymer they call “PolyfluoroEster.” Development of products based on this new polymer and field testing on real users gave the company the feedback it needed: They had a product that demonstrated real improvement over silicone-based anti-frizz products!
PolyfluoroEster, the little polymer that could
The self-purported magical component of the Living Proof line of hair products is a polymer only disclosed as “polyfluoroester”, which is fairly non-specific in terms of revealing the actual molecular structure to us. The organization is understandably extremely protective of their intellectual property, as keeping the secret ingredient under wraps will help maintain their advantage in the competitive beauty products market. However, the CurlChemist has been digging in the trenches to discover what she can, so we can make some educated guesses about its structure, how that might affect its performance on our hair, and what sorts of expectations we can have for these products.
So what is it? Well, the short answer is that it is a modified fluoropolymer, which is a synthetic polymer made up of the typical carbon-based backbone, but with fluorine molecules either attached directly onto the backbone of the polymer or suspended from the sides of the chain as substituents. The fluoropolymer most consumers are familiar with is Teflon, or poly(tetrafluoroethylene”>.

Molecular structure of Teflon (PTFE”>
PTFE, or Teflon, is prized for its unique chemical and thermal stability, its extremely low coefficient of friction, as well as its ability to act as both a water and oil repellent. It is truly “non-stick.” However, one limitation is its extremely high molecular weight (too heavy for hair”>, and another is its complete insolubility in virtually any solvent, including water. For these reasons (and others”>, polymer scientists have experimented over the years with many variants of this molecule, using different monomers and different combinations of monomers to create modified fluoropolymers and copolymers as well (polymers synthesized using mixtures of different monomers”>. There are companies who market fluorinated reactive surfactants as well.

Molecular structure of a fluorinated copolymer
Armed with all of this information, my best educated guess is that this PolyfluoroEster is possibly a fluorinated polyester (a polymer with a polyester backbone, with fluorinated substituents as pendant side groups”> or a fluorinated acrylic copolymer (similar to many of the acrylic copolymers currently used in styling products – just with a fluorine twist, if you will”>. Whatever the specific case may be (and as a polymer scientist, I am so hopeful that one day we can know the actual structure”>, it is most certain that this polymer has a significantly lower molecular weight than PTFE and most silicones used in hair care products. The marketing material of Living Proof states that these smaller molecules do not weigh down the hair, and that for this reason the consumer can use much more of the product and coat all of the hairs on her head entirely. This creates a very flat, sealed cuticle layer and forms a true protective shield against the ravages of humidity upon the hair.
PolyfluoroEster is both hydrophobic and lipophobic, meaning it is neither water soluble nor oil soluble. Different ingredients in the formulation work to help emulsify the polymer and actually get it into solution in their product. These polymers not only repel water, but also repel oils and dirt (particulate matter”> from the environment, so it becomes possible to go longer between shampoos, which helps to minimize the inevitable damage that occurs with shampooing. That is a great feature, especially for those of us always in pursuit of that elusive creature, beautiful second-day hair.
Fluoropolymers have extremely low surface energy, which allows the polymer to spread very evenly and to form a very smooth, thin film on the surface of the hair (referred to in their product literature as a microfilm shield”>. This low surface energy and smooth film formation helps to increase slip and decrease friction between adjacent hair strands. This prevents tangling and eases combing, which can be very beneficial to the health of hair. It also acts as another mechanism for frizz prevention, because it helps prevent static charge accumulation that can occur from friction between hair strands.
This polymer is reported to have a low refractive index, which is credited with giving it the ability to impart high gloss and shine. I have to admit to being perplexed by that, because silicones have a high refractive index, which is responsible for their ability to be such fantastic glossifiers. I intend to review my understanding in that area a bit and also to contact Living Proof directly, as I have a few more questions for them anyway. I will be happy to report back anything they share with me.
Is this PolyfluoroEster compatible with curly hair?
These products definitely look promising for curly-haired consumers who suffer from problems with frizz, especially in humid climates. It seems as if the product line is very gentle, with their sulfate-free shampoo and their moisturizer-rich conditioners. Their styling products look very reasonable as well. The polymer is most definitely not water soluble, so one might have concerns regarding a shampoo-free (CG”> routine. However, it is my hypothesis that due to its molecular structure, this polymer does not build up on itself, similar to amodimethicone, so many who get good results from amodimethicone-containing products might be very successful with this as well (perhaps even moreso”>. Much like amodimethicone though, I am curious does thoroughly remove it from one’s hair, as it wouldn’t seem to be susceptible to most usual surfactants. I am hoping to get more information on this from the scientists at Living Proof.
Most of the reviews I have seen for the product have been really good, so I feel that the NoFrizz line of products is very promising. Apparently, performance of this product improves with repeat applications too, so make sure you use it for a few days or longer when evaluating it on your hair. I have heard that at least one of the styling products can be quite stiff for those of us with curly hair, and that can make the hair feel brittle and dry, so be careful which product you choose and perhaps layer it with their leave-in conditioner.
The main downside of these products is their cost. However, the polymer itself is in the products at higher concentration than other polymers usually are, and it is probably quite expensive to make right now. Also, a huge amount of money has gone into the acquisition of the absolute crème de la crème of the brains in the scientific and beauty industry and into the building of state of the art laboratories. All of that comes with a price! I am hoping to try some soon myself. (Dear Santa…”>
I am really interested in these products and in this innovative new company, so I would love to get e-mails or comments below from you guys about how you feel about it after trying it.
Addendum
I had the most wonderful opportunity to speak to Jessie Vallette, a customer service representative at Living Proof, after I had completed this article. I was so pleased with the level of knowledge she had and her willingness to discuss my questions in detail. Again, my hat is off to this company. So, on to the information she shared.
Polyfluoroester is definitely completely hydrophobic, and so is not water insoluble. However, when the hair is immersed in water, some small quantities of it will come off of the hair. Immersion in water will also still cause the hair to swell and the cuticle to open, so it is still possible to clean the hair and for conditioner to do its job. They have found that their own very gentle shampoo does remove the polymer from the surface of the hair, so it is not necessary to use a sulfate-containing cleanser.
Also, she confirmed that polyfluoroester does not build up on itself once it has adsorbed onto the surface of the hair, so it seems to behave in a very similar manner as amodimethicone in that respect. Many curly-haired consumers who use a no-shampoo or low-shampoo regimen have been successful with products that contain amodimethicone for this reason. According to Jessie, this polymer should be even easier to work with, so the No Frizz line may well outperform products that contain amodimethicone when it comes to frizz-fighting.

A variety of shea butters.
Butters, oils, and waxes all come from fats that are derived from plants or animals, and have two basic components; fatty acids and alcohols. The difference between butters and oils is primarily whether or not they are solid at room and/or body temperature. Although they are both composed of groups of fatty acids, there are differences in the molecular composition and structure of butters and oils that are responsible for these differences in melting points.
Factors that determine melting point of lipids
- Molecular weight – lower-molecular-weight fatty acids have a lower melting point, so that they are liquid at room temperature or body temperature. Higher-molecular-weight fatty acids form crystalline structures that persist to higher melting points, and so they are usually solids at room temperature and higher.
- Saturated molecular structure — longer-chain fatty acids without any double bonds are straight chain molecules (like long snakes”> that are able to closely pack next to one another This close-packing induces crystallization, which requires more energy to break apart than molecules not packed together into a crystalline or semi-crystalline structure. For this reason, the melting points of these types of fatty acids are much higher. This means the “oil” will exist in a solid state at room temperature or even body temperature.
- Unsaturated molecular structure — unsaturated molecules have at least one double bond somewhere in their structure. This creates a kink or branching effect in the geometry of the molecule. This prevents unsaturated fatty acids from getting too close to one another, thereby preventing crystallization. These molecules have lots of space between themselves, which allows for more mobility of the molecules and results in a lower melting temperature. These oils may be liquid at room temperature or melt upon contact with skin.
- Stearic acid, a saturated hydrocarbon molecule with 18 carbons (relatively long-chain fatty acid”> has a melting point of 69.6°C (157.28°F”>. Oleic acid, a monounsaturated hydrocarbon molecule, has a double bond in it that creates a kink in its geometry, which makes it more difficult for adjacent molecules to pack tightly next to one another. It has a melting point of 10.5°C (50.9°F”>. Polyunsaturated acids, such as linoleic and linolenic, have multiple kinks in their chains and are liquid at very low temperatures (melt point = -5°C (23°F”> for linoleic acid”>.
- Linolenic acid, polyunsaturated fatty acid.
- Linoleic acid, polyunsaturated, omega-6 fatty acid.
- Oleic acid, monounsaturated fatty acid.
- Stearic acid, saturated fatty acid.
Clearing up some misconceptions

Various Oils
Coconut butter, avocado, almond butter and peanut butter are not actually butters, in terms of the nomenclature we are discussing. In these products, the flesh of the nut or fruit is pressed and included with the oil, which provides the food source with proteins and water, as well as fatty acids. This is not a butter in the technical sense THEN WHAT IS IT? WHAT IS THE DEFINITION OF BUTTER?, but this is a common usage in food products. Another point to keep in mind is that in some cases, the unsaturated fatty acids in the oils of these fruits and nuts are hydrogenated to create a more solid texture. This can change the properties of the product significantly.
Another bit of confusion on this topic of oils, butters, and waxes is due to misleading terminology in the nomenclature system. It is not uncommon to read assertions that emulsifying is waxy or oily and prone to build-up. In fact, emulsifying wax is not a wax at all, nor is it an oil. It is a group of ingredients (derived from fatty acids “> used as a nonionic surfactant mixture that is highly effective at facilitating mixing of oils and waxes into aqueous solutions. Specifically, it is most often these ingredients: Cetearyl Alcohol, Polysorbate 60, PEG-150 Stearate & Steareth-20. All of those components are water soluble, with the exception of the fatty alcohol. It is possible that people who dislike products containing this ingredient are actually sensitive to the oil or butter being emulsified by emulsifying wax, or they are sensitive to buildup of fatty alcohols (cetearyl alcohol”> on their hair.
Fatty acid composition of common vegetable butters and oils
| Vegetable | Butter or Oil | Fatty Acids |
|---|---|---|
| Shea | Butter |
|
| Cocoa | Butter |
|
| Mango | Butter |
|
| Wheat Germ | Oil |
|
| Avocado | Oil |
|
| Coconut | Oil |
|
| Olive | Oil |
|
| Jojoba | Oil |
|
| Almond | Oil |
|
Depending upon where it is grown and the environmental conditions of that year, as well as soil quality, shea butter can vary significantly in its ratio of stearic acid to oleic acid. This will affect its melting point, and thus, its softness. If there is a lot more oleic acid relative to stearic acid in a particular batch, it will be a much softer and oily product, and will behave somewhat differently on the hair. Processing (methods of extraction, filtering, use of heat, hydrogenation”> can also drastically affect the unsaturated oil composition, so if purchasing shea butter itself, carefully read the label so you are aware of the quality of butter you are getting. A pure shea butter contains no emulsifiers or perfumes, but is purely the mixture of fatty acids that were extracted from the fruit.

Shea butter
Incorporation of shea butter into a conditioning product involves melting it and dissolving it into an emulsifier and then mixing that into the product. Although the butter is melted and mixed into a liquid, its mixture of fatty acids should remain intact (unless high heat was used, which is not typical”>. Therefore, it is still the same “butter”, simply because the term butter is not actually very meaningful. The inherent molecular structure is unchanged.
It is also interesting to note that while coconut oil is comprised almost entirely of saturated fatty acids, it is still referred to as an oil, rather than a butter. This is due to the lower molecular weight of the major fatty acids in coconut oil, which give it a lower melting point; typically right around room temperature. This is another clue that the terms butter and oil are not always very precise or meaningful. For this reason, it is a good idea to look at the fatty acid content of a particular butter or oil you would like to try and to understand what sort of performance you might expect based upon its chemistry, rather than what it is called.
Which is best for our hair?
So, we have established that all of these butters and oils are made up of different mixtures fatty acid molecules. What specifically accounts for the varying preferences expressed by consumers? Some curly-haired people extol the virtues of butters, while others adamantly proclaim oils as their holy grail ingredient.
The major components of butters and coconut oil are one or more saturated fatty acids, while the major components of most oils are a mixture of mono- and polyunsaturated fatty acids. One must assume that the differences in performance when used as emollients for the hair are a direct result of these differences in molecular structure. This is exactly the reason, and the science is fairly straight forward.
You will recall recent discussions we have had regarding the nature of the cuticle layer of the hair. Pores in the cuticle layer (whether from damage or from its being slightly open due to being wet”> allow passage of some molecules into the cell membrane complex layer that is just beneath the cuticle scales. The fatty acids in this lipid layer act as a diffusion port that allows some fatty acids to penetrate the hair shaft. However, due to molecular structure and geometry, not all fatty acids are created equal in their ability to diffuse into the hair.

Murumuru butter
Generally, molecules with a straight chain geometry (saturated fatty acids”>, such as stearic acid, lauric acid, and palmitic acid can easily fit through the pores of the cuticle layer and slither through the CMC and into the interior of the cortex. Recent spectroscopic studies have allowed scientists to confirm that monounsaturated fatty acids are also able to readily penetrate the interior of the hair via this route. However, polyunsaturated fatty acids seem unable to penetrate into the interior of the hair at all, and remain either adsorbed onto the exterior surface of the hair or may get wedged into the cuticle layer.
Fatty acids in the interior of the hair can provide brittle hair with much-needed suppleness and elasticity. However, porosity is a very important factor to consider when using easily absorbed oils and butters. If one has very porous hair, it can absorb excessive quantities of these oils, which can lead to a host of problems. Among these could be greasy feel, dull appearance, limp hair, a swollen and open cuticle, frizz, and tangling. It can be very difficult to remove excess absorbed oils, in my experience, requiring the use of harsh surfactants, which strip the hair of its own lipids.
Oils high in polyunsaturated fatty acids may provide ease of wet combing and prevent static build up and fly-away hair. In addition, they form a barrier film on the surface of the hair, preventing moisture from escaping the interior of the hair. However, they might contribute to hair that feels greasy or sticky to the touch. Oils on the surface of the hair can also attract dirt to your hair. Another potential concern is that if these types of oils are indeed wedged into the cuticle layer at all, hair becomes vulnerable to the dangers of having a raised or rough cuticle.
My recommendation is to try small quantities of different oils and butters and carefully observe your results and preferences. Then, when you find things you really like or dislike, look into the fatty acid composition of the things you tried. This will help you determine if your hair needs saturated, monounsaturated, or polyunsaturated fatty acids, and you can then choose your products accordingly, armed with your knowledge of the underlying chemistry!
As a polymer scientist with a love for biological structures, I find hair and skin to be extremely fascinating systems. Human hair is an intricate composite structure comprised of keratin proteins, lipids, polysaccharides, water and pigment particles. All of the individual components are complex and perform very specific functions. Those of us with curly hair are concerned a lot about our hair’s texture and porosity (a popular buzz word of late”>. These two factors are primarily based upon the structure of the cuticle — the outer layer of our hair.

Fig. 1: Undamaged hair
The scanning electron microscope image in Figure 1 shows highly magnified detail of the exterior surface of a strand of human hair. The external layer is called the cuticle, and is much like bark on a tree. Both the cuticle layer and tree bark are made up of many smaller, individual pieces (called scales when referring to the cuticle”> that work together as one overall unit to perform a function. The job of the cuticle is to provide protection to the hair shaft from mechanical and thermal damage, while allowing moisture in and out as needed. The cuticle structure is an amazing work of nature, because it is strong, yet flexible, and is made up of many pieces, which allows it to act as a seal to protect the inner cortex of the hair, and yet also allows it to be permeable, or porous.
The center of the hair shaft is referred to as the cortex, and is a very complicated structure filled with many different substructures and clusters of structures made of keratin protein, lipids, and other matter. Water provides the means for the necessary hydrogen bonding between the keratin fibers to occur that is essential for the maintenance of hair strength, elasticity and integrity. Without moisture in the cortex, the hair becomes thin, frizzy, and much more prone to permanent damage and breakage. Thus, the cuticle layer performs a very important duty by protecting this delicate interior of the hair and helping it maintain the proper balance of moisture.
Structure of the Cuticle
The cuticle itself is a multi-laminate structure, like stacked sheets of paper, composed of fatty acids, proteins, and other cellular matter. Below is a description of each layer.
- Epicuticle — This surface layer of the cuticle is made up of lipids and proteins and is also found on the bottom of the stacks of layers.
- A-Layer — This layer is comprised of proteins very high (35%”> in cystine, which enables the layer to be highly crosslinked. This layer gives toughness to the hair and also provides physical protection from heat and other potential threats.
- Exocuticle — This layer has approximately 15% cystine, so it is less strong and tough than the A-Layer, but provides similar protection.
- Endocuticle — This layer contains only 3% cystine, and so is only very lightly crosslinked. This means that this layer is the only cuticle layer to swell in the presence of water. This causes the entire cuticle to swell and lift away from the hair shaft, resulting in a ruffled cuticle that allows the passage of material both into and out of the hair.
- Cuticular Cell membrane Complex (CMC”> — This layer is made up of polysaccharides and several lipids (fatty acids”>. This layer acts as the glue that holds the cuticle together and holds it to the hair shaft.
Cuticle Damage

Fig. 2: Chemically altered hair
A perfectly healthy hair that has not been exposed to harsh chemical processing, prolonged sunlight, or rough thermal and mechanical treatments (often called “virgin hair””> will have a cuticle layer such as the one shown in Figure 1. The individual keratin scales lie very flat, have fairly smooth edges, and overlap one another, forming a flat, tight sheath around the interior of the hair shaft. A hair in this condition is highly protected from the environment, retains the most moisture, and generally is more reflective, giving the hair shine and gloss. (This latter feature is variable, depending upon the color and the degree of curl of the hair”>.
Unfortunately, most of us don’t have hair protected by such a beautiful, intact cuticle. As hair ages, it is continually exposed to sunlight, water, pollution and external mechanical forces. UV radiation from sunlight can break down some of the keratin bonds and cause deterioration of scales and cause them to lose some of their structural integrity. Mechanical forces such as combing, bruising, curling, pinning up or binding the hair can all catch the edges of cuticle scales and ruffle or raise them, creating a rough surface more prone to tangling and tearing. Rough treatment can even pull cuticles off entirely.
Water causes the endocuticle layer to swell, which lifts the entire cuticle and creates a rough surface. This leaves hair more delicate and susceptible to tangling and damage from friction between adjacent hair strands or from mechanical forces. For this reason, wet hair should be treated very gently and conditioners must be used to reduce friction and combing forces. Hair exposed to high humidity should be protected by extra conditioning and anti-humectant products in order to avoid this effect, which can lead to very damaged summer hair. (Read an article about summer hair here.”>

Fig. 3: Damaged cuticle
These (scary”> images show strands that have had extensive damage done to the cuticle layer from chemical processes (coloring, perming, relaxing”>. It is evident from viewing these images that once the cuticle layer is damaged, the cortex becomes exposed and the entire hair is extremely vulnerable to virtually any threat. The best solution in these extreme cases is to have a professional stylist trim the hair.
Curly hair is more susceptible to cuticle damage due to its structure and due to its lower amount of internal moisture. As a curly hair strand bends and curls around itself, sections of the cuticle layer can become slightly raised. These individual scales can cause tangles and breakage, get caught on a comb or scrunchie and magnify the effects of other types of damage that can occur to hair in our daily lives. For this reason it is imperative that people with curly hair avoid as many damaging conditions as possible, moisturize as much as their hair will tolerate, and use products that smooth the surface of the hair. It is critical to remember that prevention of damage is always the goal. Once cuticles are damaged or removed, further damage is inevitable until that portion of the hair is removed.
Next month I will discuss how the cuticle layer and our treatment of it affects porosity and what that means for our hair.
References:
- Syed, A.N., Askar, N.A., “Structure of Hair” Powerpoint presentation, www.dralisyed.com, July 3, 2008
- Robbins, C. R., Chemical and Physical Behavior of Human Hair, Spring, 4th Editions, Dec. 14, 2001
- Gray, John, “The World of Hair”, P&G Hair Care Research Center
Email your questions to Tonya.
Polyquats are of major interest to curlies, specifically the removal of polyquats for those who avoid sulfate cleansers. Build up of any moisturizing or styling agent is a genuine problem because it can lead to limp curls, frizzy hair with a straw-like texture — and it can even lead to permanent damage in the form of breakage. For this reason, I persistently search for publications of studies done that specifically investigate this topic.
Polyquats (polyquaternium”> are polymers frequently used in hair-care products to provide conditioning benefits to the hair. The trait they all share is that they are very large molecules with periodic positive charges located at different sites along the molecule. There are many different types of these cationic polymers, with widely varying molecular structures and charge densities (amount of positive charge/molecule”>. Each specific type of polyquaternium molecule is assigned a numeric designation, such as polyquaternium-4 or polyquaternium-11.
Some of these molecules are linear, like a snake or a single piece of spaghetti, while others have many branches like a tree. Most are fairly high molecular weight (in the 500,000 — 1,000,000 grams/mole range”>, which imparts the maximum conditioning benefits. A few of the polyquats are obtained via chemical modifications of naturally-derived polymers such as guar gum and cellulose. However, the majority are completely synthetic molecules developed and tested in laboratories, typically copolymers of various monomers selected to give the polymer the final desired properties. (If one is interested in further study, these monomers are often one of the following: vinyl pyrrolidone (VP”>, diallyl dimethyl ammonium chloride (DADMAC”>, dimethylaminoethyl methacrylate, and quaternized vinylimidazole (QVI”>.”>
A BASF research group based in Germany published findings of a study they performed to compare the performance of several different common conditioning polyquats. They used several different test methods and sensory feedback tests to compare conditioning effectiveness and how easy it will be to remove removability of a common group of cationic polymers, including cationic guar gum, polyquaternium-7, polyquaternium-10, polyquaternium-11, and polyquaternium-44.
To determine conditioning effectiveness, they measured wet comb-ability of hair treated with each formula. (Wet comb-ability measures the forces required to comb through wet hair after application of the conditioning agent”>. They also surveyed consumer texture/sensory feedback on each type of polymer (typically accomplished in a small hair salon on site, where volunteers try different products on their hair and report how it feels to them”>. Atomic force microscopy was used to determine residual polyquat quantity on the hair after rinsing or washing with an anionic surfactant (such as sodium laureth sulfate”>.
The chemists reported that polyquaternium-44 led the pack by exhibiting superior conditioning benefits while simultaneously resisting build up. Cationic guar gum had the poorest performance in both regards. Polyquaternium-10 did not do as well in the conditioning efficacy analyses, but proved to also be easily removable. Polyquaternium-7 and polyquaternium-11 were somewhere in the middle of the group.
There were several interesting things to be gleaned from the behavior of polyquaterium-44. This is a completely synthetic, branched copolymer, of fairly high molecular weight, and fairly low charge density. Branched polymers like this are more coiled when in aqueous solution, unlike the more extended conformation adopted by their linear counterparts. For this reason, they are more easily deposited onto the surface of the hair, where the positively charged moieties bind to the protein surface.

Figure 1. Conformation of two different polymer structures in water — a linear polymer on the left and a branched polymer on the right.
The BASF chemists hypothesize that because of the low charge density of this particular polymer, much of the structure actually extends away from the hair surface in loops and coils. These little wiggly bits would act to reduce friction between adjacent hairs, which would reduce tangling and increase ease of comb-ability. This configuration is also credited with how easy it is to remove with a water rinse or shampoo rinse. Many cationic polymers resist removal with even the most harsh shampoos, so this is very interesting behavior.
Another research and development group observed similar behavior with a high molecular weight, branched and lightly crosslinked cationic polymer. This polymer displayed conditioning benefits superior to other cationic polymers when used in relaxer solutions. The inventors discussed data that supports that this polymer penetrated the hair cuticle due to its being open during the chemical relaxing process. Once inside, they believe it formed an elastic cushion inside the hair that provided lasting strength and elasticity to the hair. They also found that this crosslinked, non-linear material was more readily removed from the hair surface than other types of cationic conditioners, which also supports the findings of the BASF group.
The take-home message of this article is that products containing polyquat-44 will give you the best results compared to those formulated with other types of cationic polymers. It provides fantastic detangling and moisturizing benefits and detaches from the surface of the hair easily. Products with polyquat-10 are also a pretty safe choice for those on low-shampoo plans. It would be wise to keep in mind though that other polyquats as well as positively-charged silicones such as amodimethicone may be much more difficult to remove from your hair. As always, your mileage may vary, so don’t be afraid to experiment with products and ingredients!
Sources:
Hössel, Dieing, Nörenberg, Pfau, & Sander (BASF Aktiengesellschaft, Ludwigshafen, Germany”>, Conditioning Polymers in Today’s Shampoo Formulations; Efficacy, Mechanism and Test Methods, International Journal of Cosmetic Science, Vol. 22, No. 1, pg. 1-10, 2000
Woodruff, John, Formulators; it is time to widen your horizons, Paper given before the Society of Cosmetic Scientists, Chepstow, 2000
Agent: Joann Villamizar Ciba Corporation/patent Department – Tarrytown, NY, US
Inventors: Emily Crisp Bazemore, Rhonda F. Tsotsoros, Zhiqiang Song, Jianwen Mao
USPTO Application #: 20080138307, Use of high molecular weight crosslinked, water-soluble cationic polymers in hair care formulations
Email your questions to Tonya.
Curly hair can be considered as a precious cashmere sweater that requires the utmost gentle handling and care. Most of us are aware that our wavy and curly tresses have a tendency to be drier than other hair, and can be very fragile and delicate. For this reason, we have been urged to avoid using harsh detergents on our hair, with special cautions against using the “sulfates.”
This is rather sound advice with regard to a very small number of ingredients (that do happen to be quite pervasive in the formulations”>, but it is often oversimplified and extended to include other ingredients that can be useful in a haircare regimen. With that in mind, it may be useful and interesting to explore the nature of surfactants, and then specifically sulfate surfactants, how they differ from one another, and what effects these molecular differences can have on curly hair.
What is a surfactant?
A surfactant or detergent molecule is one that possesses the trait of having one distinct portion of the molecule that is polar and hydrophilic (water-loving”>, and one portion that is non-polar and hydrophobic (water-fearing”>. This dual nature enables these molecules to interact favorably with water and water-soluble molecules as well as with oils and other types of water-insoluble molecules.
In many surfactants such as sodium lauryl sulfate, the hydrophilic portion is found to exist on a terminal end of the molecule, and for this reason it is often referred to as the head group. The hydrophobic portion of these linear surfactant molecules is typically an alkyl or aryl containing chain, which is referred to as the tail group. (Figure 1″>

Figure 1. Illustration of a simple linear surfactant molecule
Surfactant molecules are classified according to the ionic charge of the hydrophilic head group. These classes consist of anionic, cationic, nonionic, and zwitterionic surfactants. The most common anionic surfactants have a sulfate (such as SLS”>, sulfonate, phosphate, or carboxylate (soaps”> functionality as the head group, while cationic surfactants are often tertiary or quaternary salts of alkyl amines (cetrimonium chloride”>. Ionic surfactants are available in salt form with an appropriate alkali metal or ammonium counterion (typically sodium or ammonium”>.

Figure 2. Illustration of a micelle
Sulfate Surfactants
Sodium lauryl sulfate is probably the most commonly used anionic surfactant in the personal-care business. It is relatively inexpensive, foams quickly and is a fairly efficient cleanser. This molecule has 12 carbon atoms in its hydrophobic tail group and has a low critical micelle concentration, which means it has relatively good cleansing capabilities.
The downside is that it can be irritating to the skin, and as we have observed, can be quite harsh to delicate, dry hair. This is less the case when the formula which contains it has sufficient conditioning agents and co-surfactants present to minimize this effect”>. It has also acquired quite a reputation (primarily due to misinformation spread through the internet”> for being a health hazard, but that topic shall have to be addressed another day.
Sodium laureth sulfate, which is a slightly larger molecule than SLS modified by the addition of ether groups, has the ability to form larger micelles and is consequently a more efficient and harsher cleanser. This is also true for ammonium lauryl sulfate, ammonium laureth sulfate, and sodium myristyl sulfate and sodium myreth sulfate. (*** please note that this is different information than I have previously provided. I am looking into it further, but SLES and ALES are less irritating to the skin and eyes, which has possibly allowed them to enjoy an erroneous reputation for being gentler to the hair as well”>.

Figure 3. Sodium lauryl sulfate (top figure”> and sodium lauryl ether sulfate. The ammonium versions of these molecules have an NH4+ counterion in place of the sodium ion.
Sodium coco or cocoyl sulfate is sometimes seen on the labels of products wishing to market themselves as natural, gentle and luxurious. It is touted for being derived from coconut oil, which is true. It is typically a combination of sodium lauryl sulfate (usually around 50%”> and sodium myristyl and palmityl sulfate (longer chain hydrocarbon tails”>. Although this is derived from coconut oil, it goes through a rigorous chemical reaction and purification process, and the result is a surfactant mixture that performs in the same manner as the ones derived from petrochemical feedstock sources.
Sulfosuccinates are another kind of sulfate surfactant seen in hair and skin-care products. These ingredients possess two hydrophobic tails, which is a very different molecular architecture than the straight-chain sulfates. Molecules of this type are not as efficient at packing into micelles and are very mild cleansing agents. For this reason, they are not excessively drying to the skin or hair and should not be of concern, even though they retain their status as “sulfates.”
Magnesium sulfate, an ingredient often used as a curl enhancer in styling products, is not a surfactant but rather an inorganic crystalline compound. This material does not strip away moisture or oils from the hair or dry the hair in the same manner as a sulfate surfactant. It can create frizzy or dry-textured hair for some users, but for different reasons that shall be discussed in an upcoming article.
The Take-Away Message:
If a shampoo formulation were merely made up of water, surfactant and preservative, it might seem pretty cut and dried to avoid most of your sulfate-based surfactants. However, in most formulations, there are co-surfactants present, such as fatty alcohols, nonionic surfactants and amphoteric surfactants (cocamidropropyl betaine”> — many of which help to reduce the drying tendency of the cleanser. Also, most formulations contain additives such as moisturizing oils and conditoning polymers that can help prevent damage to the hair. Additionally, the composition by weight of each component in the formulation is important to the overall performance of the product.
If you are attempting to avoid harsh shampoos, it is probably best to make sure you find a product that contains milder surfactants, lots of moisturizing agents, and little, if any of the linear chain sulfates. However, if you like to use them occasionally as I do, look for ones that have SLS or SLES lower down the ingredient list, and make sure the list contains other mild co-surfactants and moisturizing agents.
Email your questions to Tonya.
Vinegar has been used as a health and beauty aid for thousands of years to brighten skin, soften hair and improve health. Now, as with all things natural, simple, cheap, and “green,” it is experiencing resurgence in its popularity as consumers become more conscious of the effects products may have on their health and on the environment.
Many marketers of natural soaps recommend the use of apple cider vinegar in particular as a rinse aid when using their product. Lorraine Massey suggests using it as a cleanser and rinse aid in her book “Curly Girl.” And many other web sites and message boards extol its virtues as well.
Here, I’ll discuss how best to use vinegar to care for your hair.
The Unique Benefits of Apple Cider Vinegar
Apple cider vinegar is made by adding sugar, yeast, and bacteria to apple cider. This process ferments the cider into an ethanol solution, and then the bacteria convert it into an acetic acid solution. The cloudy and sometimes slimy portion of apple cider vinegar is actually dead cells of bacteria and yeast (yum!”>, sometimes referred to as “mother of vinegar.”
Apple cider vinegar is highly prized in the natural-products community, both for its reported health benefits and its extra nutrients that can help hair or skin. That may be the reason it is most often the rinse recommended for hair. However, current research does not show there to be any appreciable amounts of vitamins, minerals, enzymes or amino acids present in apple cider vinegar.
Other types of vinegar are made from wine, malt, corn, rice, coconut, sugar cane, or even beer. The pH for all the different vinegars range from about 2.5 to 3.5, depending upon the concentration of acetic acid (and the other acidic byproducts which can occur in the process, such as citric acid and tartaric acid”>. When it comes to use as a hair tonic or rinse, there really should be no performance difference between any of type of vinegar, so you can pick the one you prefer for reasons of smell or price.

Vinegar’s Benefits to the Hair
Vinegar is very useful as a rinse for the hair for several reasons. Acetic acid is a mild chelating agent, so it can be useful in removing mineral deposits on the hair that accumulate over time due to impurities in the air and hard water. The clarifying properties of ACV and other vinegars also extend to the removal of accumulated sebum or other waxy buildup from products. When used for this purpose, it is important to use a greater concentration of vinegar (25 to 50 percent”>. Rub it into the scalp and leave it on the hair for a few minutes, which gives it time to work.
Another excellent application for vinegar is to use it in conjunction with soap/shampoo bars, as a means for the prevention of build up of hard water–induced soap scum, which can be devastating to the condition of your hair.
Vinegar is also just a great pH adjuster for the hair when used as a rinse in a dilute aqueous solution. It is essential that your hair be the proper pH after cleansing and conditioning, as this affects the overall health, appearance, and condition of your hair. For this reason, most modern shampoos and conditioners are “pH balanced,” meaning they are adjusted to a pH very close to that of the hair in its healthiest state.
The relationship between pH and hair
Human is hair is made up of strands of fibers made of keratin protein, which contain the amino acids glycine, alanine, and cysteine. Cysteine contains sulfur, and is responsible for the disulfide bonds in hair. These contribute to the overall strength and elasticity of hair strands as well as to curl formation. Proteins are made up of many amino acids linked together into a chain, and then gathered and folded into other structures. Due to their chemical structure, proteins have the capability to possess either a positive or a negative charge, depending upon the local environment. There exists one pH (at a given temperature”>, where the molecule is completely uncharged or neutral, and this is known as the isoelectric point. The isoelectric point for human hair optimally is a pH value of 4.0 to 4.5.
Hair that is at its isoelectric point has a tight structure and a sealed flat cuticle layer on the outside of the strand. Things that can raise the pH of our hair are structural damage from processing or rough treatment; use of basic solutions on the hair such as perming solutions, relaxers, or baking soda; and soap bars or soap containing detergents. Hair at a higher pH is negatively charged, and has a more swollen and porous structure. It also has lifted, ruffled cuticles that contribute to a dull appearance, frizzy character, tendency to become tangled, and a higher propensity for breakage.
Since vinegar is acidic, with a pH between 2.5 and 3.5 (depending upon type and concentration”>, using it in a dilute solution as a final rinse for your hair or as a rinse prior to conditioning is an excellent way to lower the pH of your hair to the isoelectric point or slightly below it. This allows the hair to be sealed flat with a smooth outer layer of interconnected cuticle scales. This has the effect of making hair very shiny, with fewer tangles, more bounce and greater mangeability. To obtain maximum benefit from your rinse, I recommend preparing your solutions from water that has been filtered or softened. It is important to use vinegar carefully and in conjunction with a good conditioner, as it can be somewhat drying to the hair if used frequently or in too high a concentration. Overall, it is a fantastic and affordable addition to any hair-care routine.
Email your questions to Tonya.

When Bumble and bumble launched its Curl Conscious campaign, the company turned to curlies for its inspiration. We talked to Bumble and bumble Senior Vice President Artistic Director Howard McLaren about what went into developing the innovative campaign.
NaturallyCurly: How did you come up with idea behind the campaign?
McLaren: We were really inspired by how passionate our curly customers are about their hair. Curly haired people have such a specific point of view, and we really noticed how knowledgeable they are about their hair and its needs. They are in a class by themselves. We really wanted to honor the curl community with a campaign that celebrated not just curly hair but the entire culture behind this specific hair type.
NaturallyCurly: What was the feeling you hoped to get across with the campaign?
McLaren: We wanted the campaign to be celebratory and to honor all different sorts of textures and personalities. If you look at the photos of our campaign, they are all in the same soft fashion colors of the packaging and they evoke a very free-spirited vibe. We were inspired by the curl models, and clients we’ve met and worked with throughout the years and perceive them as very strong individuals who have a sense of belong to a larger community that’s unified by their hair.
NaturallyCurly: What is the meaning of the paisley in the campaign?
McLaren: The paisley print of the product packaging was inspired by a great story out of India. Long ago, during harvest time, farmers would dip the sides of their fists in ink and mark their grain bags to denote ownership. The kidney-shaped fist print evolved into the paisley print. We thought the paisley would be the perfect complement to our curl products since no two fist prints are alike, just like no two curls are alike.
NaturallyCurly: How did you find the models that appear in your campaign?
McLaren: We chose Los Angeles for our campaign shoot because we encountered these great kids who loved and embraced their curly hair. We cast the models via Facebook and Model Mayhem and also traveled to Los Angeles and asked our stylists to tell their friends in the area to stop by. After a mass casting of 150 models, we narrowed it down to 50 and selected 12 key models who we really felt represented the wide spectrum of the curl community. We loved their individual looks and the way they presented themselves.
NaturallyCurly: Tell me how you came up with the styles for the photo shoot.
McLaren: We took a look at the models — their face shape, hair texture, etc. — and created a haircut for each of them that was tailored to their individual look. That’s what cutting curly hair is all about. Curl textures vary so drastically. It’s really important that each head of hair is uniquely addressed.
NaturallyCurly: You chose to work with stylist Rolando Beauchamp on the shoot. Why?
McLaren: Rolando is such a versatile and experienced stylist who can really work with all hair types. He’s had quite a bit of experience working with curly hair throughout his career, and we knew he’d really make the shoot amazing. He’s our leading editorial stylist and has a relationship with Bumble and bumble that stretches back through much of his career. You’ve seen his work in magazines such as “Italian Vogue,” “Purple,” “Dazed and Confused,” The New York Times “Style” magazine and “Marie Clarie” as well as on the runway for such designers as Karen Walker and Vivienne Westwood.
NaturallyCurly: What is your philosphy behind curly hair?
McLaren: Curly hair is amazing, but it has special needs. When caring for, styling and cutting this texture, you have to be very present and careful. All three of these areas (caring for, cutting and styling”> need to be addressed. It all starts with a great cut, so that’s why Bumble has just inaugurated its first curl workshop this past November so we can educate our network stylists to the special needs of curly hair. Our curriculum really emphasizes individuality. Since there are so many different textures within the curl category plus so many different styling preferences among clients, it’s really important to ask questions so you get a feel for what needs should be addressed. My philosophy really is that there is no tried and true rules. Just be sure you ask questions. Particularly with a curly client who is often so knowledgeable and involved with their hair, cutting and styling curly hair is a collaborative experience.
NaturallyCurly: Let’s talk about the products. How did you tweak the Curl Conscious line?
McLaren: We realized that the two regimens we tried to enforce in the original line weren’t working. No curls are alike, and while the line was successful, people struggled with understanding which camp they fit in. We decided to focus on giving as many customizable options as possible when restaging the line.
We simplified the cleansers and conditioners with universal formulas. With a better understanding of curly hair, we realized we really didn’t need so many options. The majority of our clients preferred the shampoo and conditioner for fine-to-medium hair, no matter what their curl type was. So we made a few updates to the formulas.
We had a lot of people asking for something that was even more moisturizing than the medium-to-thick conditioner, which led us to create the Nourishing Masque. The client can control how often they use it, whether that’s once a week or once a day.
Styling was the strongest part of the line, but people wanted more options. So we created the Holding Foam for hold and control and the Reactivating Mist to revive curls throughout the day and on no-shampoo days.
With the packaging, we wanted it to complement the feel of the campaign. With the clear naming and curl differentiations as well as the colorful, hand-drawn paisley texture, the idea was that they have a distinctive identity that can work together or stand alone.
It seems that naturally curly folks have a love/hate relationship with glycerin. There is a lot of information, and misinformation, about this seemingly simple substance. I thought I’d shed some light on the chemical makeup of glycerin and how it affects curly hair specifically.
So, what is it
Glycerin is an alcohol, known also by the chemical names glycerol and 1,2,3, propane triol. It can be obtained via hydrolysis of naturally occurring plant or vegetable fats (triglycerides”> or via chemical synthesis from petrochemicals. It is a conditioning alcohol similar to other conditioning alcohols, but it has three very hydrophilic hydroxyl (-OH”> groups as opposed to a single one. Because of this, glycerin is far more water soluble than some of the other conditioning alcohols such as lauryl alcohol and cetearyl alcohol.
Glycerol has been used for many years in cosmetics and personal care, and has been found to be a remarkable moisturizer for the skin and hair. In fact, new studies have shown that it has amazing abilities to actually aid in repair and regeneration of skin cells.

Figure 1
Chemical structure of glycerol
Glycerol obtained from naturally occurring fats is a product of chemically breaking down triglyceridesinto their fatty acid components. Triglycerides are fat molecules comprised of a glycerol backbone with three arm-like appendages of fatty acids bound to the glycerol via an ester linkage. These three fatty acid chains can all be the same exact molecule (such as stearic acid”>, or can all be different from one another (one stearic, one lauric, one palmitic – for example”>. Hydrolysis of the ester bond results in the production of glycerol and three fatty acid molecules.
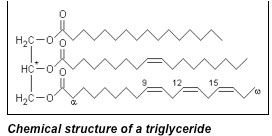
Figure 2
Glycerin can come from multiple natural sources because triglycerides can be derived from animals or vegetables. Some examples of vegetable sources would be coconut oil and shea butter. Typically, if you are purchasing glycerin, the label will say if it is from a vegetable source of glycerin. However, a multi-ingredient finished product may not disclose the source of glycerin (animal, vegetable, or synthetic”> unless it is a marketing point for the brand.
Synthetic glycerin has received some publicity as being a potential health hazard, and many consumers prefer to avoid it. It is typically produced from the starting material epichlorohydrin, which is a toxic chemical that is classified as a probable carcinogen. Of concern is the presence of trace remnants of epichlorohydrin or another potentially carcinogenic contaminant such as 1,4 dioxane. So, while synthetic glycerin provides the same benefits to your hair as glycerin derived naturally, there may be sufficient risks associated with it to warrant choosing only vegetable or animal-derived glycerin.
Properties of Glycerin:
Glycerin is an alcohol and is soluble in water and also in other alcohols. It is insoluble in oils, but is able to dissolve many oils and can be used as an emulsifier for adding oils into a formulation. In its pure form, it is odorless and colorless, but has a sweet taste. It has a thick viscosity and is clear, so it is frequently added to formulations for its moisturizing and viscosity-modifying properties. Adding it to a formula is an inexpensive way to impart a thick, velvety texture to a product, a property typically valued by consumers. It is a highly effective moisturizer and humectant for skin and hair. Its thick viscosity and high boiling point are what make it an effective curl-definer and frizz minimizer (in the right climate”>.
Glycerin is a relatively small molecule compared to many moisturizers, and it contains three hydroxyl groups. This high molecular density of hydrophilic groups makes it an extremely hygroscopic molecule that absorbs water from its surrounding environment. It does this to such a high degree that it will raise a blister if applied in an undiluted state to the skin. If it were applied to hair in such a concentrated state, it could strip all of the moisture from the interior of the hair.
However, when used in a diluted form, glycerin can be a great moisturizer and humectant for the hair. Care should be taken to use it in environments of moderate humidity. If the climate is very hot and humid, glycerin will absorb a lot of moisture from the air and cause the hair to swell, raising the cuticle and disrupting curl pattern, creating coarse, frizzy hair. In weather that is extremely dry, glycerin will seek out moisture from your hair and actually dehydrate it, which can cause damage and breakage. Read more about this.
Concerns and precautions
Many heat-styling techniques can generate sufficient heat to boil the water inside the hair shaft, which can cause terrible breakage. One way to prevent or minimize this problem is to coat the hair with an emollient that seals in the moisture and that does not transfer the heat from the appliance to the hair as readily. Unfortunately, glycerin conducts thermal energy pretty efficiently (it transfers heat readily to the hair”>, especially when compared to silicones, proteins, and polyquaternium conditioning ingredients. For that reason, use glycerin sparingly and in combination with a more insulating and protective moisturizer when using any sort of heated drying or styling treatments.
One of the biggest concerns I have seen expressed about glycerin is that it might remove or strip color from the hair. Many people with chemically dyed hair avoid it almost unilaterally. The truth of the matter is that glycerin is a reasonably good solvent for many types of molecules. It can dissolve and grab unbound dye molecules that are easily accessible near the exterior of the hair.
This is relevant in a few different cases. Users of permanent chemical dyes should exercise some caution in the initial days following processing. Hair that has been freshly colored with a permanent chemical dye is susceptible to loss of some colorant molecules because the cuticle may not have completely re-sealed. However, it is not cause for concern after the first washing because the colorant molecules have penetrated into the cortex of the hair shaft, the cuticle is sealed, and any excess on the surface is washed away.
Users of semi-permanent dyes or colors notorious for being short-lived (such as shades of red”> may be wise to be extremely cautious regarding the use of glycerin in their hair. This is because these types of dye molecules are too large to penetrate the hair and reside predominantly on the exterior of the hair shaft. Glycerin can easily dissolve and remove these colors and accelerate the inevitable fading process.
To Sum it Up
Glycerin is a water-soluble conditioning alcohol and is an extremely effective moisturizer and humectant. This means that all the usual things to be aware of regarding humectants, curly hair, and the weather are applicable when using glycerin.
Its viscosity and clarity make it a great ingredient to give definition to curls and to smooth the fly-away hair.
Use it in combination with another conditioning agent if planning to use heat-styling methods.
Do not use glycerin right after using a permanent dye on the hair, and consider avoiding it if you use semi-permanent or red color on your hair.

This month I want to address a topic I see pop up on the message boards pretty frequently: use of homemade or diluted products.
I pretty frequently read of products such as hair conditioners or styling products being diluted with water and used as daily leave-in treatments. Many people also enjoy making some of their own products, using natural ingredients such as honey, aloe vera, water, and various oils often found in the kitchen. The results obtained from these can be wonderful. But it is important to exercise a bit of caution regarding spoilage.
Products purchased off the shelf have been very carefully formulated and pretty exhaustively tested in laboratories by microbiologists in order to ensure that the correct amount of preservative is present in order to prevent growth of fungi, mold, and bacteria (sounds yummy, huh?”>. These antimicrobial compounds also act as antioxidants, protecting the chemical stability of the product. The optimal level of preservative per unit volume is calculated and used in order to be just sufficient for that particular bottle in ordinary use conditions for a specific period of time. It is important to not use too much preservative because preservatives themselves can be harmful to humans or cause allergic reactions. Therefore, there is not typically an excess of preservative in most products.
For this reason, when a product is diluted with water (especially tap water”> and placed into a non-sterile container (which is most typical in any household”>, the preservative level is reduced by as much as 100 times or more. This diluted product is then often stored in the hot and damp conditions of a bathroom. These conditions are perfect for the breeding of all sorts of living entities that just love to live in water and eat organic molecules (such as those found in your conditioner”>.

Lacking the inhibition of a safe amount of preservative, microorganisms can grow unchecked and create a genuine health risk for anyone using them. Another potential effect of significantly diminished preservative concentration is degradation of the molecules in the product itself via oxidation from environmental exposure. This can cause development of noxious odors or the formation of potentially dangerous degradation products. At best, it can simply render your product ineffective.
The risk of your being unwittingly exposed to organisms or degradation products is most high when the solution is already cloudy due to the presence of an oil component (such as in a conditioner or styling product”>, which obscures your ability to observe growth at an early stage. Also, due to the presence of fragrance, one might not detect spoilage by smell in the early stages, either. By the time you are able to detect a problem, you could possibly have been applying a contaminated product for a few days.
My recommendation if you like to use diluted conditioner or other products on your hair is to do one of the following (and always with clean hands, boiled water, and a sterilized container”>:
- Make up a small amount of solution daily or every other day.
- Make up enough solution to last for one week and store it in the refrigerator when not in use.
- Make up enough solution to use for 1-2 weeks and add either natural or synthetic preservative drops to it in an amount specified in the literature. (Synthetic preservative mixtures, such as Kathon, can be purchased from a chemistry or cosmetic ingredients source, and natural preservatives such as grapefruit seed extract can be found at most health food stores”>. I would personally still store this mixture in the refrigerator when not in use and discard after a couple of weeks.
The same basic rules would apply when making a natural homemade concoction to treat your hair. If there will be water involved (in other words, it is not a pure oil product”>, then proper precautions regarding sterility and preservation should be taken to ensure your own safety.
Recently, I had a question come into my box I found very interesting. The person was curious why the shampoo she was using still generated lather, despite the fact that it did not contain sodium lauryl sulfate or sodium laureth sulfate. I immediately thought “Oh! What a wonderful topic for an article this month!” I thought we could discuss the nature of bubbles, lather, and foam, what causes them to occur, and what ingredients are likely to enhance or minimize their formation.
Imagine my surprise when I started doing a little research to refresh my understanding of bubble formation and found this topic to be an area of much intense research, most of which seemed to be far over my head! Many physicists, astronomers and chemists devote their entire lives attempting to develop a fundamental understanding of bubbles, foam, and lather. So, while feeling a little daunted, I decided to carry on and do my own rudimentary analysis of the subject.
What is a bubble?
A bubble is a ball of gas encapsulated by a microscopically thin film of liquid. The formation of a bubble is made possible by the fact that the surface of a liquid behaves much like a skin. This behavior is due to intermolecular forces.
Water molecules possess a high degree of polarity, due to the presence of two small electropositive hydrogen atoms on one end of the molecule and a single, larger electronegative oxygen atom at the other end. These polar molecules tend to be attracted to one another, and arrange themselves so that there is plenty of dipole-dipole interaction, allowing the hydrogen atoms to align with oxygen atoms, and vice versa.
This phenomenon is known as hydrogen bonding, and while it is technically a physical interaction, the strength of these interactions can be quite high. Hydrogen bonding is responsible for many of the unique properties of water such as its high boiling point, relative to its molecular weight, its relatively high density when in liquid form, and the very interesting fact that it is less dense as a solid than as a liquid.
Within the bulk of the liquid, molecules are in constant motion and are continually encountering one another and then moving into another portion of the liquid. This constant motion means that the dipole-dipole forces exerted on each molecule come from all directions, so the forces are evened out. However, at the air-water interface of a solution, there are not significant levels of intermolecular forces coming from the direction of the air phase. For this reason, the water molecules at the surface experience a much stronger pull in the side-to-side and underneath directions. This has the effect of creating stronger resultant forces (cohesive forces”> between water molecules at the surface, which creates a phenomenon known as surface tension. Surface tension is what creates skin-like behavior at the surface of the water.

Figure 1. This is a snapshot of a computer simulation of H2O molecules in liquid water. The dashed blue lines represent hydrogen bonding between the molecules as they flow through the liquid. (Image courtesy of Wikipedia”>

So what does all this have to do with bubbles? This surface tension is what makes the formation of bubbles possible. When air (or another gas”> is forced into water (via agitation, blowing, rapid flow from a faucet, or injection via any other means”> and bubbles form, surface tension in the thin layer of liquid that forms the skin of the bubbles draws the bubble tightly into the shape, which has the least surface area to the highest volume (which is a sphere”>, and this is called a minimal surface structure.
Pure water will form bubbles, which I am sure you have noticed when filling a glass of water from the faucet. However, you have probably also noticed that these bubbles don’t stick around for long. This is actually a result of the extremely high surface tension of water, due to all those hydrogen bonding forces. The addition of soap or surfactant molecules to an aqueous solution acts to bring down the surface tension of the water (a phenomenon discussed briefly in other articles I have written for this site”>. By lowering the energy at the air-water contact point, soap molecules act to stabilize this interface. That is why the presence of soap or surfactant molecules in water will act to stabilize bubbles that form.
Soap, Lather and Foam
When there is sufficient soap present to stabilize bubbles, multiple bubbles can begin to meet and come together to form a complex structure called foam.
When three or more bubbles join together, the walls always meet at angles of exactly 120°. Nature can be amazingly precise! Eventually this becomes a complex structure comprised of hexagonal cells, which looks a lot like a beehive. (This type of hexagonal structure is one of very low energy, a state highly favored in nature”>. This conglomerate of bubbles is called foam, which is really all that lather is.
Certain surfactants, due to the simplicity of their structure, very readily form and stabilize bubbles and foams. For this reason, in the formulating world, they are often referred to as foaming agents. They typically produce lots of lather when they are present in a shampoo or skin cleaner and are frequently included in products in order to boost this effect, as it is considered to be desirable by many consumers.
Sodium lauryl sulfate and sodium lauryl ether sulfate (SLS and SLES”> are both well-known for producing lots of lather when used in shampoos. These two ingredients are also classified as harsh cleansers, especially for those of us prone to damage done by the effects of stripping oils away from the hair. For this reason, it has become a commonly held belief that foam and lather necessarily correlate with potentially damaging effects.
However, many of the gentler cleansers, such as cocamidopropyl betaine are also used as foam boosters, and will create lather when a product containing them is used to cleanse the hair. While it is true that some of the extremely mild, larger molecule cleansers will only foam minimally or not at all (a fact that is disconcerting to many consumers”>, most surfactants will cause lather formation to some extent. Other ingredients in the shampoo, as well the hardness of water being used will affect foam formation also (most often adversely”>.
It is important when evaluating a shampoo to remember that some foaming and lather formation are actually not indicative of a cleanser being harsh. It is best to avoid products that contain SLS and SLES if your hair is dry and delicate. However, if you choose a shampoo that has an apparently gentle ingredients list, but you still get some lather, fear not! Conversely, if you enjoy a little lather when washing your hair but your gentle shampoo doesn’t do this for you, don’t worry that your hair won’t be clean. Foaming and lather formation are not the main phenomenon by which one should gauge the effectiveness or gentleness of their product.
Sidney Perkowitz, professor of physics at Emory University and the author of “Universal Foam,” describes foams as examples of soft matter: They don’t flow freely like a true liquid, but neither are they a crystalline solid like a diamond. “We’re very good at explaining hard matter like crystals; the entire semiconductor industry is based on them,” he says. “Soft matter seems to tell us a lot more about nature and biology.”
Email your questions to Tonya.
We get a lot of questions about this strangely-named ingredient: simethicone. What exactly is it, and why is it found in some hair care products? What function does it perform, and is it water soluble? If it isn’t water soluble, what cleanser is required for removal from the hair?
There’s always a lot of confusion surrounding simethicone. It’s name sounds similar to dimethicone, but it is just dissimilar enough as to make it unclear as to what its nature truly us.
Simethicone
Simethicone is a mixture of poly(dimethylsiloxane”>, the silicone known in cosmetics and personal-care products as dimethicone, and silica gel — hence the name: silica + dimethicone, shortened to simethicone.
This mixture is often supplied to personal care product manufacturers as an emulsion, stabilized with nonionic surfactants, pH modifiers, and preservatives. The dimethicone content can be as high as 30 percent of the simethicone mixture, but of course is never at such a high concentration level in the final product. Simethicone is probably most well-known by the consumer as an orally-administered medication with the purpose of reducing gas in the intestinal tract. Many colicky babies are given drops of this over-the-counter medication by desperate, tired mamas. (It may be interesting to note that studies are indicative that this medicine actually produces no change in outcome when compared to giving the baby nothing at all”>.In hair-care products, such as conditioners, it is used to reduce foaming in a product by minimizing the surface tension of bubbles that are formed by agitation of the container. This minimization of surface tension results in the formation of fewer and larger bubbles, rather than a foamy layer of many densely packed smaller bubbles. The addition of simethicone to product formulations may also modify the viscosity of the product in interesting ways, such as causing the formation of gels when the solution is at rest in the container, but that flow easily when the container is squeezed (think toothpaste and hair gels”>. The dimethicone in the mixture will also deposit onto the surface of the hair and act as an emollient and impart gloss to the hair. Clearly, the addition of simethicone to a product formulation can provide multiple enhancements to product performance.
Simethicone
It is my personal belief that even some of the natural, nonionic surfactants are up to the job of removing dimethicone from hair. As always though, it is important to remember that everyone’s hair reacts differently to products due to a variety of factors such as hair type, hair condition, water hardness, and environmental conditions, so you may have to try a few things to find what works best for you!
I hope this article helps bring some clarity to this one tiny portion of the sometimes murky waters of silicone ingredients, mixtures containing silicones, and everything in between.
Email your questions to Tonya.
As we move into spring and summer — our favorite seasons for fun outdoor activities — we face challenges with our curly hair that are unique to the climate and activities. Here is a great reader question that I will use as a springboard (no pun intended”> to offer some curly hair tips for protecting your curls in warmer, more humid weather. This column is written more from my scientist perspective than an ingredients-chemistry perspective, in case you were wondering!
Q: All winter, my hair behaved. My hair is thin, but there is lots of it! It loves mousse and a little conditioner. Now that it’s humid, my hair is misbehaving. What do I do? More conditioner? Gel instead of mousse? I like to elongate my curls as much as possible, they can get tight. Any help would be much appreciated!
A: There’s no denying that the change change in seasons can be tough on our hair care routines. Products and processes that were working so well suddenly seem to have the opposite effect. Often, at the heart of these issues is a change in the environmental moisture content (humidity”>. High humidity is especially harsh on curly hair.
The reason for its susceptibility to humidity fluctuations lies in the physical structure of curly hair. Straight hair, undamaged by environmental or treatment factors, has a protective outer layer of cuticle scales that overlap and lie fairly flat against one another. Curly hair, even in very good condition, is much more porous because those cuticle scales do not always lie flat. This porosity allows more water to migrate out of curly hair into the environment in dry weather (not good”>, and also allows more moisture from the environment to migrate into the cortex of the hair strands in humid weather (also not good”>.
Absorption of water into the hair from the environment works to disrupt hydrogen bonds between adjacent hairs, which can diminish curl clumping and definition and lead to flyaway hair and a frizzy appearance. This absorption of moisture can also cause the hair shaft to swell and ruffle up the cuticles a bit, which can lead to tangling, an unpleasant overall texture, and more frizz. Hair strands swollen with water, and with ruffled cuticles because of water absorption, are also more delicate, and can more easily be damaged or broken.
 Here are two scanning electron micrograph images of single human hair strands. The one on the left is smooth and shows a cuticle layer in excellent condition. The image to the right shows a damaged cuticle layer, with raised scales, chipped cuticle scales, and spots where the cuticle has been completely removed.
Here are two scanning electron micrograph images of single human hair strands. The one on the left is smooth and shows a cuticle layer in excellent condition. The image to the right shows a damaged cuticle layer, with raised scales, chipped cuticle scales, and spots where the cuticle has been completely removed.
Absorption of water from the air into the central core of each hair strand is much more pronounced in hair that has even greater porosity because of raised or damaged cuticles. Chemical treatments, such as coloring and perming, as well as the use of thermal styling tools (hair dryers, hot rollers, curling irons”> or rough combing or brushing, frequent exposure to sunshine, and even tossing and turning in our sleep can all damage these cuticles and increase the porosity of curly hair.
The process also occurs to a greater extent if the hair needs hydration. For this reason, it is very important to maintain hair in the best condition possible at all times, but especially in warm and humid weather. In warm, wet weather, keep your hair very well-moisturized and use a good leave-in conditioner, but try to avoid using products that include humectants in their ingredients, as these can aggravate problems with humidity-induced frizz. Styling gels seem to perform better in this type of weather than mousse. The polymer styling agents in gels form a nice protective film around the hair, which helps maintain the style throughout the day.
Curly hair tips for lovely summer locks:
- Trim hair regularly
- Avoid using thermal drying and styling techniques
- Gently finger comb or use a wide-toothed comb on wet hair that is saturated with conditioner
- Be selective about chemical treatments on the hair — minimize the frequency of such processes
- Avoid frequent exposure to direct sunlight — wear a hat or use a leave-in product containing sunscreens for the hair
- Use generous amounts of moisturizing products, both in the shower and as leave-in treatments. (This reduces diffusion of moisture into and out of the hair”>
- Consider using a low-viscosity, easily spreadable hair gel as your primary styling agent for the summer.
Guar and its derivatives are used in many shampoos, conditioners, and styling products. These ingredients always seemed pretty innocuous to me, so I was surprised when I found a few curlies who did not like the performance of products that contained them. It seemed like a good idea to put on my scientific detective cap and delve more deeply into the topic of guar and its derivatives, such as guar hydroxypropyltrimonium chloride.
What is Guar?
Guar gum is a naturally occurring biopolymer obtained from the seeds of the guar plant (Cyampopis tetragonolobus,”>. Grown in semi-arid regions, this biopolymer belongs to a specific group of polysaccharides known as galactomannans. Guar is a water-soluble polymer due to the presence of many hydroxyl (-OH”> groups along its backbone.

The guar plant
Depending upon its concentration, guar can thicken an aqueous solution considerably, and also has the ability to form gels. For this reason it is often used in shampoos, conditioners, and hair gels as a viscosity modifier (thickener”>. It also has the property of being shear-thinning, which means its viscosity decreases when mechanical shear is applied. The result is that the product can be poured or squeezed out of the container with little force, but it comes out slowly enough to be thick and luxurious. Application to the hair feels smooth and silky, as the shear forces applied allow for easy spreadability. For these reasons, guar is often found in foods as well, such as ice cream, where it increases the viscosity and improves the texture.
Hydroxypropyl Guar
This is a derivative of guar that has been hydrophobically modified by the addition of hydrophobic moieties along the

Figure 1. Chemical structure of guar, source: Martin Chaplin BSc PhD, http://www.lsbu.ac.uk/water/hygua.html
backbone of the polymer. The typical degree of substitution is not very high because it is undesirable that the molecules become insoluble in water. The addition of these few hydrophobes along the polymer allows it to work as an even more efficient thickener via a mechanism known as hydrophobic interaction. Products using this polymer at very low concentrations are relying upon its ability to provide excellent texture and viscosity modification.
What is guar hydroxypropyltrimonium chloride?
Guar hydroxypropyltrimonium chloride (INCI name”>, also known as 2-hydroxy-3-(trimethylammonium”>propyl ether chloride by strict organic nomenclature, is a quaternized derivative (cationically modified”> version of the guar polymer. This high molecular weight polymer retains its original structure, but has side groups added along the backbone of the chain that possess a positive charge.
This cationic polymer has all of the thickening capabilities of the original guar gum, but also has the additional benefits of substantivity to keratin (hair protein,”> and improved moisture retention, as well as improved wet and dry compatibility. It has also been found to decrease irritation when used in body washes and shampoos which contain certain anionic surfactants that are known irritants. Another benefit of cationic conditioners is that they tend to resist buildup. They are used at relatively low concentrations in formulations (see Table 1 below,”> because not very much is necessary to achieve the desired effects.

Figure 2. Chemical structure of Guar hydroxypropyltrimonium chloride, courtesy Hercules Incorporated, Aqualon Division
So why do some folks dislike this ingredient?
The reason some curly haired consumers dislike guar and its derivatives may be the same reason others enjoy it. The presence of so many hydroxyl (-OH”> groups along the polymer chain makes it very water soluble, but also makes it an excellent humectant. It can attract water to the hair and retain large quantities of it. In some climates and for some hair, this is a highly desirable trait.
But for other hair types and for those living in hot, humid climates, it can result in frizzy hair with a coarse texture. As with most ingredients, it is necessary to experiment with products to find what works best for your own hair.
Q: Some of the ingredients in my products have names that look a lot like names for silicones. What are they? Are they silicones? Should I be concerned about how they may affect my hair if I am avoiding strong surfactants (sulfates”> in my hair care routine?
A: It gets confusing, doesn’t it? There are so many different ingredients approved for use in personal care products, and sometimes their INCI names (International Nomenclature of Cosmetic Ingredients”> start to sound very similar. Chemical nomenclature (organic or otherwise”> is a pretty in-depth topic, and there are several different naming conventions of which INCI is only one. Rather than tackling that beast, let’s simply take a look at some of the ingredients frequently confused with silicones and try to get some clarity around this issue (at least with a few ingredients!”>.
First of all, what is a silicone?
A silicone is a polymer or oligomer with an inorganic (non carbon-based”> backbone, typically with organic pendant groups. The structures of silicone polymers or cyclic oligomers (such as cyclopentasiloxane, cyclomethicone”> always include silicone atoms and oxygen atoms as part of the backbone of the molecule. These molecules are used in a wide variety of products, typically for their superior conditioning effects and their ability to impart gloss and shine.
Many curly-haired consumers who have delicate, dry hair avoid frequent use of shampoos containing strong surfactants. People who adhere to a low sulfate or sulfate free shampoo routine often avoid products containing silicones because they are almost always water insoluble (with a few exceptions”> and can cause problematic buildup on the surface of the hair if not removed via a shampoo. For this reason, they read labels of all the products they use and occasionally experience confusion when an ingredient has a name similar to the silicone family of ingredients.

Figure 1. Generic structure of a silicone molecule – note the presence of silicon and oxygen atoms.
Following are some examples of ingredients whose names sometimes raise red flags for those consumers concerned about the presence of silicones in their products:
5-Bromo-5-Nitro-1,3-Dioxane
5-bromo-5-nitro-1,3-dioxane (trade name Bronidox”> is a six-membered cyclic ether, a member of a group of molecules known as –oxanes. This name is easily confused with the very similar notation used for many silicones: polydimthylsiloxane. Bronidox is an antimicrobial and preservative that is especially effective against yeast and fungi. It is slightly less soluble in water than alcohols, but it is present in hair product formulations in such low percentages that there is no concern whatsoever about buildup on the hair, regardless of hair-care routine (no shampoo, low shampoo, etc.”>

Figure 2. Structure of Bronidox
Isothiazolinones: (methylisothiazolinone and methylchloroisothiazolinone”>
Methylisothiazolinone (MIT”> and methylchloroisothiazolinone are preservatives that are usually sold and used as a mixture (trade name: Kathon CG”>. They are part of the ketone family, a group of organic compounds, and the –one at the end of their name can be confused with the –one found in the names of some silicones. These chemicals are highly effective against yeasts, molds, and both gram negative and gram positive bacteria. However, they are considered to be very strong allergens and irritants of skin and membrane tissue. Some studies have also led to questions regarding potential cytotoxicity and neurotoxicity (although the FDA and CTFA do currently state that the products we use containing these chemicals are safe”>. Safety considerations aside, these preservatives are water soluble. Even if they were not, they would not be present in appreciable enough amounts to be a source of build up.

Figure 3. Structure of methylisothiazolinone
Isododecane, Isohexadecane
These ingredients are branched alkanes (-ane”>, which are molecules comprised only of carbon and hydrogen. Isodocane and isohexadecane are small, branched molecules, and are chemically very pure due to their synthetic origins. These hydrocarbon olefins are not petroleum distillation byproducts as many other raw materials often are, but are synthesized via highly advanced processes from very pure starting material.
They are used in cosmetics due to their low toxicity, low skin irritation, no color and no odor. Isododecane can be used as s solvent for higher molecular weight water insoluble components such as silicones. Isohexadecane is valued for its non-greasy or tacky feel and its ability to impart a silky, smooth texture to skin and hair. It has excellent spreadability for both skin and hair also. Neither of these molecules is a silicone, but they are both insoluble in water. Due to their fairly simple and compact hydrocarbon structure, I would not expect problems with buildup if one were using a conditioner cleansing routine.
So what does all this tell me?
Hopefully we have shed some light on a few ingredients that get confused with silicones from time to time and helped you understand the differences between them and silicones and (more importantly”> whether they are okay for you to use with your particular hair routine. The bigger take-away message is that many of the ingredients listed on cosmetic packages can be strikingly similar and certainly confusing! When in doubt, look it up, ask a friend, or ask the chemist! I am always happy to answer your questions.

